God is a powerful God (e.g. Job 36:5 and Rom. 13:1).1 He does anything He pleases (Psa. 115:3), for there is no limit to his power (Gen. 18:14; Jer. 32:17, 27; Mat. 19:26; Luke 1:37).2 One of his names is Almighty (e.g. Gen. 17:1 and Rev. 21:22).3 By His power He created the universe (Jer. 10:12; 51:15), and by His power He holds the universe together (Heb. 1:3). The Almighty God is the source of every power, whether it be a power of the physical world or a power of authority (Rom. 13:1).
The following article is an excerpt from Devotional Biology: Learning to Worship the Creator of Organisms, Chapter 12.1-12.3, pgs. 253-260. The views expressed reflect those of the author, and not necessarily those of New Creation.
Physical World Energy as Illustration
As ubiquitous as God’s power is, His power is also invisible. Yet, since God wants us to understand His invisible attributes, He illustrated those attributes in the physical creation (Rom. 1:20). God illustrated His power by creating the physical world in such a way that energy is required for anything to happen in the universe. And God allowed the universe to be active or dynamic by creating an enormous amount of energy in the universe and placing it in a wide variety of forms. Energy can be put into storage, released from storage, converted from one form of energy to another form of energy, and/or used to do make things happen.

As in the remainder of the creation, energy also plays an important role in the biological world. Everything we do requires energy. We need energy to move, to grow, to speak, to breathe, to circulate our blood, to repair our molecules, to digest food, and even to use our brains. Inside cells, energy is needed for a host of cellular activities (such as building macromolecules from monomers and breaking down damaged molecules into monomers). Consequently, at every moment of every day each of our trillions of cells is storing, transforming, and/or using energy. And what is true in each cell of our bodies is true in every organism and each cell of each organism. All cells are active at all times in storing, transforming and/or using energy.
Cellular Energy Metabolism: Biology’s Power Source
Metabolism (Greek metabolē, meaning ‘change’) refers to all the chemical reactions in cells that transform molecules from one form into another (and that is every single reaction!). Besides reactions that build molecules (anabolism) and reactions that break molecules down (catabolism), metabolism also includes energy metabolism reactions that store or release energy, so that the cell can do all that a cell must do.
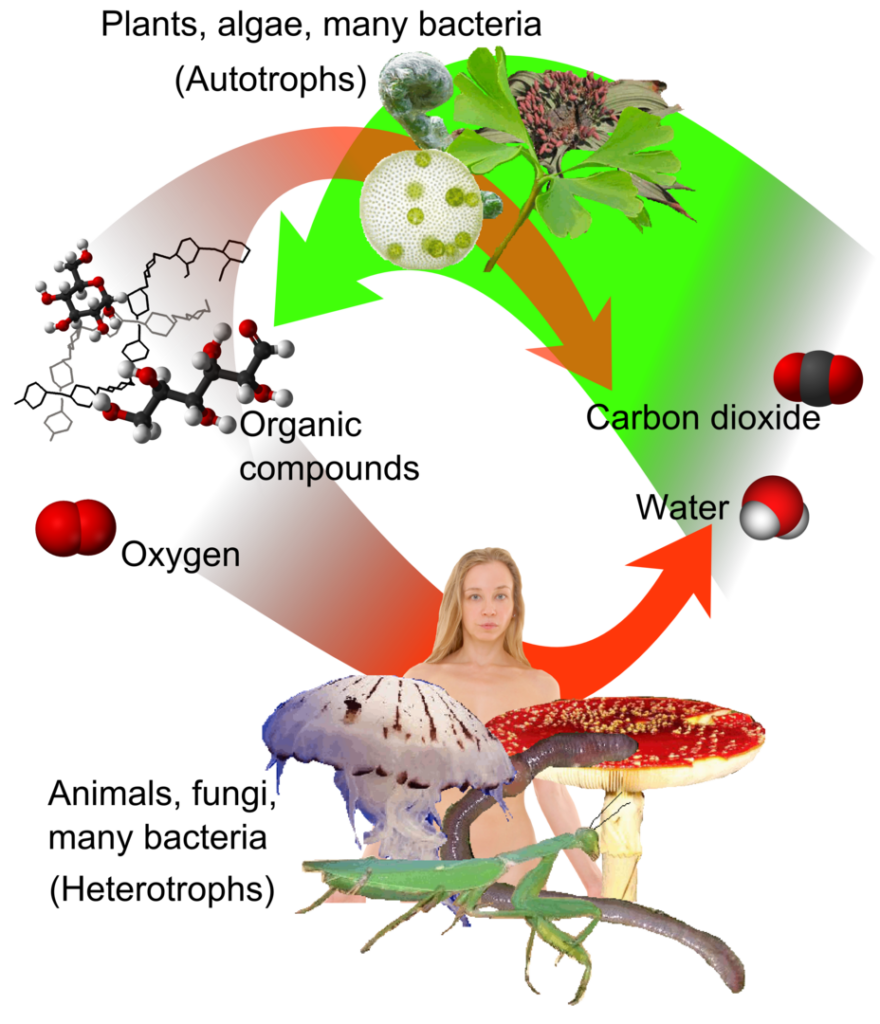
Different organisms get the energy they need in different ways. Autotrophs [Gr. eauton: ‘self’ + trophe: ‘nutrition’] can get their energy from the non-biological world around them. Autotrophs are producers and include many bacteria and most of the algae and plants. The most well-known autotrophs (most plants and algae) get their energy from sunlight, so they are called photoautotrophs.
Many bacteria, in contrast, are chemoautotrophs, since they get their energy from chemistry—more specifically inorganic molecules such as hydrogen sulfide (H2S), elemental sulfur (S), ferrous iron (Fe++), hydrogen gas (H2), or ammonia (NH3). Chemo autotrophs play important roles in biogeochemical cycles. Autotrophs—both photoautotrophs and chemoautotrophs—produce the food ultimately consumed by all organisms. Consequently, many autotrophs are designed to take the energy they acquire and store it in organic molecules—both for themselves and other organisms.
Heterotrophs [Gr. heteros: ‘different’ + trophe: ‘nutrition’] get their energy from organic molecules (such as the molecules created by autotrophs to store energy). Heterotrophs are consumers and include most of the animals, fungi, and protozoa. Most of these heterotrophs use oxygen to extract energy from organic molecules in an energy metabolism process called aerobic respiration.
Other heterotrophs release molecular energy without using oxygen. Fermentation is probably the most well-known type of oxygen-free energy metabolism. Lactic acid bacteria, for example, ferment sugars into lactic acid. Humans use lactic acid bacteria to produce cheese, yogurt, buttermilk, sauerkraut, corned beef, and salami. In another type of fermentation, yeast ferment sugars into carbon dioxide and ethyl alcohol. For example, a certain type of yeast, leaven, is added to bread dough, to ‘raise’ the dough with the carbon dioxide generated in the fermentation process. Grain or fruit is fermented with other types of yeast to create beer, wine, cider, and other alcoholic beverages. In yet another type of fermentation, acetic acid bacteria ferment ethyl alcohol into acetic acid. Acetic acid fermentation is used to produce vinegar.
Even the simplest example of energy metabolism is a very complex process. There are so many reactions involved that even well-studied examples are not completely understood. And, in the case of many an organism, its energy metabolism process is not understood at all. It is not the purpose of this text to examine energy metabolism in detail. Rather, we will choose only two well-known examples and provide only the simplest of introductions—merely enough to provide a ‘taste’ of what is involved in cellular metabolism.
Photosynthesis
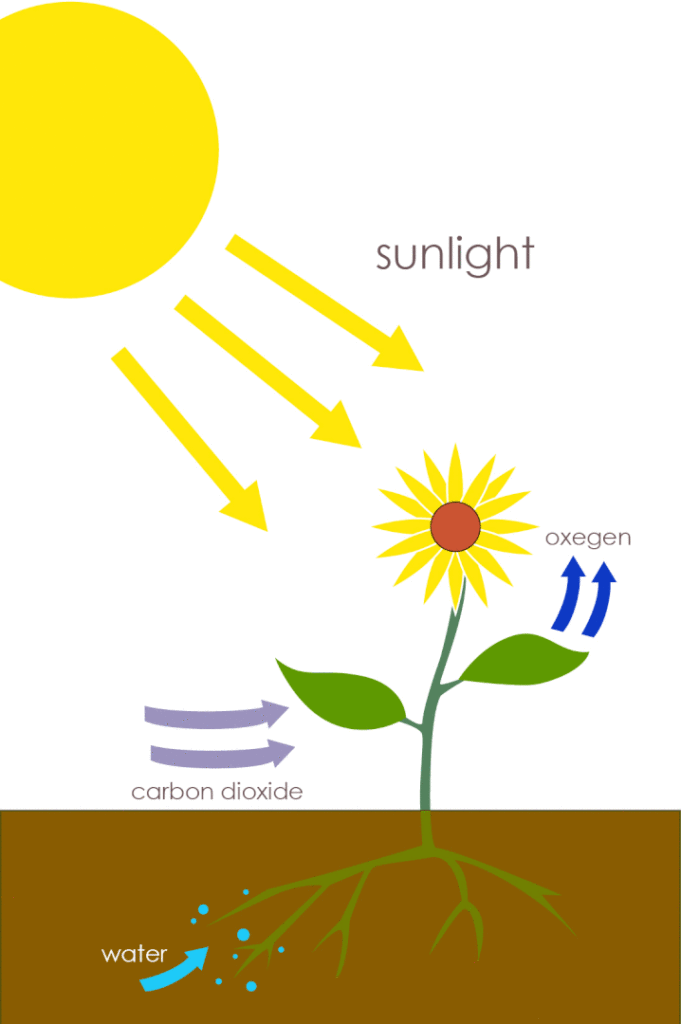
Most of the energy that circulates through land and water ecosystems ultimately comes from the sun through a metabolism process known as photosynthesis. In plants and green algae, photosynthesis occurs in cell organelles known as chloroplasts. Photosynthesis is extremely complicated, involving somewhere around 500 chemical steps. When all these chemical reactions are ‘added’ together, the result is the overall chemical reaction of 6CO2 + 6H2O + sun energy ➔ C6H12O6 + 6O2 [C6H12O6 is the formula for a monosaccharide—a sugar monomer].
In other words, ‘photo’ ‘synthesis’ is using light (‘photons’) from the sun for the ‘synthesis’ (or assembling) of sugar molecules. Referring back to the overall reaction, carbon dioxide from the atmosphere, water from the soil, and energy from sunlight are combined to build sugar, releasing oxygen gas in the process.
Pigments
Sunlight warms the earth. One does not have to stand in the sunlight long to realize that sunlight contains energy. And, although sunlight on a white surface looks ‘white’, passing that same sunlight through a prism demonstrates that ‘white’ light is actually made of all the colors of the rainbow, and a white surface is actually one that reflects all the colors. A black substance is one that absorbs all the colors, reflecting none of them. This is why black objects get so warm in the sun. A pigment is something that absorbs one or more of the colors and reflects the rest. A red pigment, for example, is something that reflects red and absorbs all the other colors. Similarly, a blue pigment reflects blue and absorbs the others. Plants and green algae are green because the pigments of photosynthesis (chlorophylls), absorb the red and blue ends of the spectrum and reflect green. Different sets of pigments—such as are found in different photoautotrophs—are colored differently (thus the common names of ‘red algae’, ‘brown algae’, ‘yellow-green algae’, ‘golden algae’, and ‘cyanobacteria’).
Light-Dependent Reactions

In green plants, the hundreds of steps of photosynthesis can be grouped into two sets of reactions. The first set of reactions requires light, so they are called the light-dependent reactions. The end result of these reactions is the production of the energy and electrons that are needed for the second set of reactions. Among the light-dependent reactions, pigments capture sunlight energy and release electrons carrying that energy. Each energy-carrying electron is released into an electron transport system (ETS), which passes the electron from one molecule to another, releasing carefully measured amounts of energy to accomplish specific tasks. Energy from one ETS is used to break water molecules into oxygen gas, electrons, and hydrogen ions (2H2O ➔ O2 + 4e– 4H+). The oxygen is rejected as waste.
In the case of the electrons, some are used to replace those that are released from the pigments, while others are carried to the light-independent reactions in special molecules called NADPH (Nicotinamide Adenine Dinucleotide Phosphate). The hydrogen ions are pumped into the innermost compartment of the chloroplast using energy from a second ETS. The concentration of ions is built up for a reason similar to why water is stored behind a hydroelectric dam. When the water is released from behind a dam, it flows downhill due to gravity, rotating components of an electric generator to generate electricity.
In a similar manner, when hydrogen ions are released from the innermost compartment, the hydrogen ions flow out of the compartment due to the second law of thermodynamics, rotating components of an ATP synthase protein so as to generate ATP.4 Some of the ATP is used to supply energy for the cell, while the remainder is passed on to the light independent reactions to be stored in sugar molecules.
LIGHT-INDEPENDENT REACTIONS

The second set of photosynthesis reactions does not require light, so they are called light independent reactions. In most plants, these reactions occur in the same chloroplasts in the same cells. In some plants, however, the light-dependent and light-independent reactions occur in chloroplasts of different cells and/or different times of the day. Light-independent reactions collect carbon dioxide from the atmosphere and electrons (NADPH) from the light-dependent reactions so as to store energy (ATP) from the light-dependent reactions in molecules of glucose. Central to these light-independent reactions is a metabolic cycle.
Humans have discovered that machines are often most efficient when they are designed to operate in a cyclical fashion. If the machine is designed never to stop, but to continue through a series of steps to constantly return to the same first step, then the machine not only never stops, but does not have to waste energy to start up again when stopped. In an automobile engine, for example, the burning of gas is used to move a piston and turn an axle. If two or more pistons are attached to the same axle at different points in their respective cycles of motion, the burning of gas at one piston will not only turn the axle, but also reset a second piston to its beginning position. Burning of gas in the second piston will then turn the axle and reset the first piston to its first position. Although some of the energy released by burning the gas is used to reset the piston or pistons that are not firing, it is much less energy than would be required to get the engine going again from a stop if it had to restart every time the piston finished burning its gas.
Cycles tend to maximize the efficiency of a process. Many metabolic processes include a metabolic cycle, probably to maximize the efficiency of metabolic processes. In most metabolic cycles, an organic molecule is modified, step by step, through a series of reactions. In the last step the molecule is returned to its original form. The metabolic cycle in the light-independent reactions of photosynthesis is called the Calvin cycle. In the course of one ‘turn’ of the Calvin cycle, one carbon dioxide molecule is fixed (i.e. combined with an organic molecule so that it is useable by organisms).
Every six times around the continuously-running cycle, six carbons are fixed into (usually) a glucose or fructose molecule. Fructose and glucose are the most important sugars in most fruits. These two sugars are used to build a wide variety of carbohydrate molecules. For example, a fructose and glucose can combine to form the disaccharide sucrose—what is usually known as table sugar. Also, a large number of glucose molecules can be joined to produce starch or cellulose.
Tree Design
The unique characteristics of water combined with the process of photosynthesis created substantial constraints on the design of land plants. Photosynthesis requires sunlight, water, and carbon dioxide, and must get rid of oxygen. Although water is most available to the plant beneath the earth’s surface, sunlight is only available above the earth’s surface. This is why most plants draw their water from underground and transport the water above ground, where photosynthesis occurs.
An important part of the process is acquiring the necessary water. Since water enters the plant through exterior membranes, plant roots must increase their surface area as fast as the amount of photosynthesis increases in the plant. As a plant increases in size, the roots must become more branched, root cells must produce more microscopic hairs, and more symbiotic relationships must be developed with mycorrhizal fungi. Roots are specially designed to acquire the water needed for photosynthesis. Then the water needs to be transported to the leaves.
The most efficient way to transport water is in a tube with a circular cross-section. Due to capillary action—a unique characteristic of water—the narrower the tube, the farther up water will travel without energy needed to pump it. In plants, water is transported in extremely narrow, circular-cross-sectioned tubes, called tracheids. These cells are tube-shaped and are placed end to end from the roots to the upper leaves. Water can travel several feet by capillary action. Water can travel the remainder of the way up the tracheids by taking advantage of the flexible hydrogen bonds between adjacent water molecules. It is as if water molecules were attached to each other with springs.
In a leaf, as a water molecule is taken into a cell for photosynthesis or is evaporated from the surface of the leaf, the hydrogen bond between that water molecule and the next one down is stretched. When the bond is stretched enough, it pulls up the next water molecule, stretching the bond between that molecule and the third one down the line. In this manner, each water molecule from the leaf to the root is pulled up one molecule at a time and one position at a time.
The flexibility of the hydrogen bonds between water molecules make it possible for an impossibly heavy column of water to be drawn up to the leaves of a plant.5 In the course of a single day scores of gallons of water can be drawn up hundreds of feet from roots deep in the ground without the plant having to expend any energy in the process. Trunks, stems, branches, and leaf veins are specially designed with these tracheids to transport water from the roots to the leaves. Since photosynthesis requires sunlight, it would be most efficient if it occurs in very thin (so light can penetrate), flat surfaces (for maximum surface area).
This explains why most of the green chlorophyll is found in thin, flat leaves. When leaves are surrounded by generally dry air, their large surface area should lead to rapid evaporation of the water brought up from the roots. Chloroplast-containing cells are thus protected from evaporation by being surrounded by small, tightly packed epidermal cells and a transparent, waxy lipid layer. Then, in order to allow carbon dioxide in, and oxygen out, there are special pairs of guard cells that can open and close around a passageway called a stoma (pl. stomata). Guard cells act as doors through the epidermis and control the entry and exit of carbon dioxide and water. Leaves also, then, are specially designed to allow as much photosynthesis to occur as possible. In short, the entire plant, from roots to stems to leaves, is specially designed for photosynthesis.
Aerobic Respiration
For the average person, the word ‘respiration’ is probably only used to refer to breathing. As we respire, we inhale oxygen and exhale carbon dioxide. We do this because each cell of our body is using oxygen and getting rid of carbon dioxide in the process of cellular respiration. In cellular respiration our cells get energy by breaking down organic molecules (like sugars, fats, and proteins).
In human cells, cellular respiration utilized the energy metabolism process known as aerobic respiration. Aerobic respiration uses oxygen and releases carbon dioxide as a waste product. We have to continually breathe in oxygen to supply the ten trillion cells of our bodies with the oxygen they need for aerobic respiration. The carbon dioxide produced by the aerobic respiration of our ten trillion cells is the carbon dioxide we must continually exhale. And it is not just humans that utilize aerobic respiration. All animals use aerobic respiration. Even plants use aerobic respiration when photosynthesis is not occurring.
Glycolysis
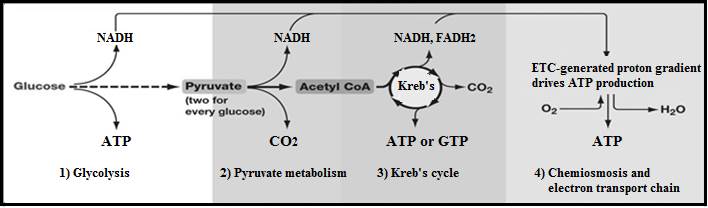
The first step in obtaining energy from sugar is not actually part of the aerobic respiration process. Because it does not require either a special organelle or oxygen, it can occur in most cells—even in cells that are unable to perform aerobic respiration. This first step is called glycolysis (glycose: glucose + lysis: break down), and it occurs in the cytoplasm. 6-carbon sugar molecules such as glucose are broken into two 3-carbon pyruvate molecules and two electrons (carried in NADH). Although it takes two ATPs to break each monosaccharide, four ATPs are created in the process. The net gain is two ATPs (4 ATPs minus 2 ATPs). In organisms that use oxygen to get energy out of molecules, the pyruvates and electrons pass into mitochondria for aerobic respiration. Other forms of energy metabolism (like fermentation) utilize the pyruvates and electrons in other ways.
How Aerobic Respiration Works
Very little of the energy found in a monosaccharide is released in the process of glycolysis. Aerobic respiration can extract a lot more energy from the pyruvates. Aerobic respiration occurs in the mitochondrion, an organelle found in almost all eukaryotic cells. The first step occurs in the innermost compartment of the mitochondrion, where each pyruvate is broken into a carbon dioxide molecule, a 2-carbon acetyl group, and an electron (stored in NADH). The carbon dioxide is released as waste. The acetyl group is combined with coenzyme A to produce acetyl-CoA.
The acetyl-CoA then enters a cyclical metabolism process known as the Krebs cycle (aka citric acid cycle). Since each ‘turn’ of the Krebs cycle breaks down one acetyl group, two ‘turns’ of the Krebs cycle completes the breakdown of the original monosaccharide. These two turns produce four CO2 molecules, ten electrons (stored in 6 NADHs and 2 FADH2s), four hydrogen ions (stored in two FADH2s), and two ATPs. The electrons are then passed on to electron transport systems in the innermost membrane and the released energy is used to pump hydrogen ions into the outer compartment of the mitochondrion. These hydrogen ions are then used to generate ATP in a similar fashion to how it was done in chloroplasts (hydrogen ions released from the outer compartment turn an ATP synthase protein). This produces an additional 32 ATP.
Considering all the steps of both glycolysis and aerobic respiration, the result is more or less the inverse of photosynthesis: C6H12O6 + 6O2 ➔ 6CO2 + 6H20 + 36ATP. Notice that carbon dioxide and water are waste products. Also notice how much more efficient aerobic respiration is than glycolysis. Aerobic respiration gets 36 ATP from a glucose molecule whereas glycolysis only gets 2 ATP.
Lipid & Protein Respiration
Energy can be derived from just about any organic molecule. If, for example, blood sugar (glucose) levels get too low, fat cells in the human body break triglycerides into glycerol and fatty acid molecules. The liver converts glycerol into a molecule that enters glycolysis of most cells. The fatty acids are taken directly into body cells and broken down into a bunch of acetyl CoAs. If the level of both glucose and triglycerides is low, the body can also break down protein.
The Origin of Cellular Metabolism

The processes of energy metabolism have all the earmarks of having been designed by the God described in Scripture. First, energy metabolism is astonishingly complex. Photosynthesis is so complex that we’re not even sure exactly how many chemical steps there are, and of the hundreds of steps that are known, only dozens are well understood. Every energy metabolism process is many times more complex than processes that occur spontaneously (or naturally).
Second, not only are there many steps in energy metabolism, but the many steps interact with extraordinary efficiency. For example, although scientists have attempted to simulate photosynthesis, the simulated systems are nowhere near as efficient as the systems in the biological world.
Third, even some of the smaller subsystems of energy metabolism look very much like humanly designed systems. For example, hydrogen ions generate ATP in a fashion similar to how hydroelectric dams are designed to generate electricity from the flow of water.
Fourth, there is the matter of the extremely small size of chloroplasts. The entire process of photosynthesis occurs within an object about a millionth of a meter in diameter. It is hard enough to imagine humans generating something as efficient as is photosynthesis, it is many times harder to imagine humans doing it on the head of pin! In the worldview of naturalism, there is no natural process capable of generating the complexity of energy metabolism. Rather, it looks like energy metabolism was designed. At the same time, since the complexity, efficiency, and size of the processes of energy metabolism exceeds the human ability to understand and duplicate, the designer of energy metabolism appears to be substantially more capable than humanity. The God of Scripture has the wisdom and manipulative ability to design such systems. And, His desire to illustrate His invisible power gives Him reason to do so.
Cellular Metabolism: Our Responsibility to God

As a lawn is mowed, thousands of blades of grass are cut. Each blade contains millions of microscopic cells capturing energy from sunlight in a system so complex and efficient that it could only have been designed by the God described in Scripture. Every green plant is evidence of that Creator and ought to remind us of the God Who created it. The same is true of algae in water ecosystems. The amazing design of photosynthesis not only provides the food for the animals of the world, but provides the oxygen they need. At the same time, carbon dioxide exhaled by animals is used for photosynthesis in plants. The creation of both plants and animals creates a well-balanced system. This should evoke worship of God because of His wisdom and awesome power.
The huge variety of biological activities that occur in the world are all powered by metabolism. This power was provided by the God of power. This should remind us how capable God is. With the Almighty, nothing He desires to do is impossible for Him, and nothing He desires us to do is impossible if we rely upon Him. Furthermore, the essential nature of energy metabolism processes in biology should remind us of how essential it is for us to derive our spiritual energy from Him.
One of the reasons God created biological metabolism was to give us a visible and finite illustration of the invisible and infinite power of God. Because He did, study of biological metabolism can improve our personal understanding of God and draw us closer to Him. To help us fulfill our priestly role, God not only created us to respond to illustrations of His nature, He also gave us the Holy Spirit to lead us into truth. Because of this, contemplation of biological metabolism can stimulate us to worship God, glorify God, and bring others into our worship of God.
Learn More About Cellular Energy
Footnotes
- Further references indicating that God is a powerful God include Deu. 7:21, I Chr. 29:11-12, Job 36:5, Job. 37:23, Psa. 24:8, Psa. 50:1, Psa. 62:11, Psa. 132:2 & 5, Psa. 147:5, Neh. 9:32, Isa. 1:24, Isa. 9:6, Isa. 10:21, Isa. 30:29, Isa. 40:26, Isa. 49:26, Isa. 60:16, Jer. 32:17-19, Jer. 51:15, Nahum 1:3, Hab. 1:12, Zeph. 3:17, Matt. 28:18, Eph. 1:19, Heb. 1:3, Jude 25, and Rev. 7:12. ↩︎
- There are, of course, a number of things that God cannot do and will not choose to do. He cannot do anything that is contrary to His nature. Because of His righteousness, God cannot sin. Because He is truth, He cannot lie. Because He is life, God cannot die. And so it continues. God does whatever He wishes to do, but will never to choose to do anything contrary to His own nature. ↩︎
- Further references to the Almighty God include Gen. 28:3, Gen. 35:11, Gen. 43:14, Gen. 48:3, Ex. 6:3, Ruth 1:20, Psa. 68:14, Psa. 91:1, Isa. 13:6, Eze. 1:24, Eze. 10:5, Joel 1:15, II Cor. 6:18, Rev. 1:8, Rev. 4:8, Rev. 11:17, Rev. 15:3, Rev. 16:7 & 14, and Rev. 19:15. Many more references are found in the book of Job (e.g. 5:17; 6:4 & 14; 8:3 & 5; 11:7; 13:3; 15:25; 21:15, 20; 22:3 & 17 & 23 & 25-26; 23:16; 24:1; 27:2 & 10-11 & 13; 29:5; 31:2 & 35; 32:8; 33:4; 34:10 & 12; 35:13; 37:23; and 40:2). ↩︎
- All cellular machinery runs on one form of energy—the energy stored in ATP (adenosine triphosphate). The molecular machines of the cell have been built to run on ATP in a manner analogous to how many of the appliances of a home have been built to run on electricity. ↩︎
- This is analogous to how a locomotive can move an impossibly heavy train. If all the cars of a train were solidly connected, locomotives would not be able to get a train to move. Rather, the connections between cars are designed with flexibility, so that even when connected, train cars can move back and forth a small distance without engaging (i.e. without pushing or pulling the next car). When it is time to move a train, the locomotive in front of the train first moves in reverse, pushing all the cars of the train together. Then, the locomotive accelerates forward as fast as possible. The flexibility in the connection allows the locomotive to gain some momentum before it begins to pull the first car. For a moment thereafter, the locomotive is pulling only one car while that first car gains some momentum of its own. Then the first car engages with the second, and so it goes until the entire train is moving—a train which is too heavy for a single locomotive to move if all the cars had to be moved at once. ↩︎