Short answer: no.
Long answer: It’s still no, but let me explain.
The following article has been reblogged with permission from Todd’s Blog. The views expressed reflect those of the author, and not necessarily those of New Creation.
As a biochemist by training, I’ve been interested in research on the origin of life for a long time. I’m not talking about God speaking creatures into existence, either. This research begins with the assumption that life emerged naturally from nonliving chemicals. You might remember from high school biology hearing about Stanley Miller’s experiment, where he zapped some ammonia, methane, and water vapor to make simple amino acids. That experiment essentially jump-started the field of origin of life research, where scientists try all sorts of weird conditions to see if they can make biochemicals using random processes.
This research has not been successful. Oh, it’s definitely made progress of sorts. Different sorts of chemicals have been formed, and we’ve found interesting organic compounds in space. But no one has successfully generated a living system from any of these experiments. Knowing what I do about biochemistry, I’m deeply skeptical that they ever will. At a chemical level, life isn’t just a simple bag of ingredients. It’s not even a bag of ingredients put together with the right recipe. Life is at minimum a system of ongoing, interconnecting reactions that are simultaneously isolated from the surrounding environment and constantly interacting with that same environment. Life is way more than that, but that description captures some of the essential features of what physical life on this planet is.
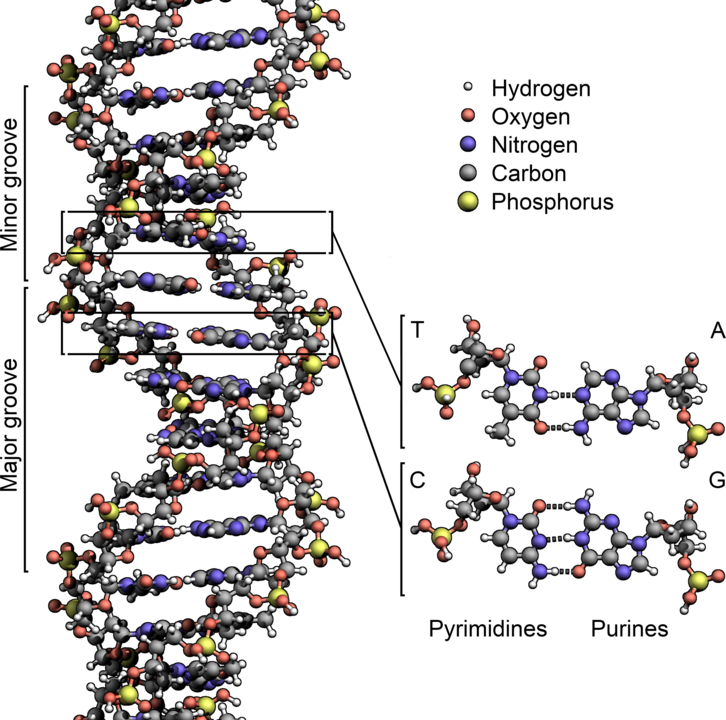
It’s the biochemical interconnections that makes life resist any simple route to random formation. For example, the central metabolic processes of life all depend on each other to function. To pick a random starting point (the interconnections assure there is no starting point), your cells all make proteins to carry out chemical functions. Digesting food, converting energy, and interacting with other cells or the environment are just some of the functions that proteins do. Proteins are made by the cell using a ribosome, which is a giant molecular complex consisting of RNA and other proteins. Where does the RNA come from? It’s made from DNA using, you guessed it, proteins. Where does the DNA come from? It’s made from pre-existing DNA by proteins. Where does the energy come from to power this system? In our cells, it’s mostly the digestion of sugar (especially glucose) that we eat. How do we break down glucose and capture its energy? Cells use proteins that are made using the energy from the breakdown of glucose. There is no absolute beginning in this metabolic network, a place where we can say, Life starts here. It’s all got to be there at once.
Yes, I am aware that there are lots of accretionary models for the origin of modern life and metabolism, and what I’ve described above is purportedly the latest iteration of billions of years of development. These models include the RNA world and other such ideas that try to simplify metabolism, to identify the minimal reactions that are necessary to sustain what we call life so that the rest of the world of living things can evolve. The problem with that is that every living thing we have today operates on the full DNA-RNA-protein-fueled-by-sugar metabolism. There are no RNA-based organisms, and we don’t have any historical evidence (i.e., fossils) that such a system ever existed. These hypotheses are just hypotheses.
Despite all this lack of progress (i.e. failure), I’m always amused at the breathless headlines that accompany the latest discoveries in the quest to unlock the origin of life. This week is no different. SciTechDaily describes the latest research as The Fountain of Life: Scientists Uncover the “Chemistry Behind the Origin of Life.” Popular Science tells us, Here’s how life on Earth might have formed out of thin air and water! Vice trots out the hilarious old chestnut, Scientists Made a Breakthrough on Life’s Origin and It Could Change Everything. Sorry, Vice. Very little has changed in light of this work.
What is this shocking new work? A new paper from Holden and colleagues describes the production of something called polypeptides using tiny water droplets. The problem they’re trying to address is the longstanding question of “polymerization,” which is the set of reactions that build up complex biological molecules from their simplest precursors. Miller showed that he could generate amino acids, the building blocks of proteins, randomly in his experiment, but that was barely the first step. Typical proteins contain hundreds of amino acids all stuck together in a row. Amino acids form these “polypeptides” by a chemical reaction that releases water. That makes an interesting conundrum: How do you make polypeptides in water, when the vast excess of water would tend to break down the polypeptides? Cells overcome this using a special array of protein and RNA molecules (the ribosome and associated enzymatic pathways), but how could it happen without a cell making it happen? That’s what Holden and colleagues want to address.
They built on lots of previous research that showed that really interesting chemistry can happen spontaneously on the surface of tiny water droplets. In their experiments they used two amino acids, glycine and alanine, to show that they could spontaneously stick them together in the right way in tiny water droplets. They produced polypeptides of as much as six amino acids in a row using this method, and they suggested that this could overcome the polymerization problem, especially since water droplets are common when waves hit the shore.
Does this unlock the origin of life? No. Does this explain how life began out of water and thin air? No. Does this change everything??? No. This is more like trying to explain the Empire State Building by showing how steel can be made from iron. It’s barely a blip. An important blip for sure, but a blip.
This is usually how it goes, too. Headlines would have you believe living cells originated spontaneously in some scientist’s test tube, but the reality is just a simple, minor bit of interesting chemistry that probably doesn’t make the unguided origin of life any more plausible than it was.
God made living things, and he made them resistant to any other explanation. That’s just how biochemistry works. Don’t let the headlines fool you.
Footnotes
Holden et al. 2022. Aqueous microdroplets enable abiotic synthesis and chain extension of unique peptide isomers from free amino acids. PNAS 119 (42) e2212642119.











A major point naturalists ignore or miss is a “warm little pond,” (darwins words) “prebiotic soup,” (haldane , oparin) or whatever was exposed to all 98 naturally occurring elements or at least 50-60 of the more common ones. Miller Urey experiments used 3 compounds and 1 element that provided just carbon, oxygen, hydrogen and carbon. They left out sulfur which 2 amino acids have. My point is all the experimental evidence naturalists claim start out with just the combinations of the 6 elements that they know the building blocks are made of. The warm little pond didn’t “know” this. I’m willing to bet if anyone has started with all 6 expecting to produce a significant amount or anything but a mess. Much worse than any reported miller urey type experiments?